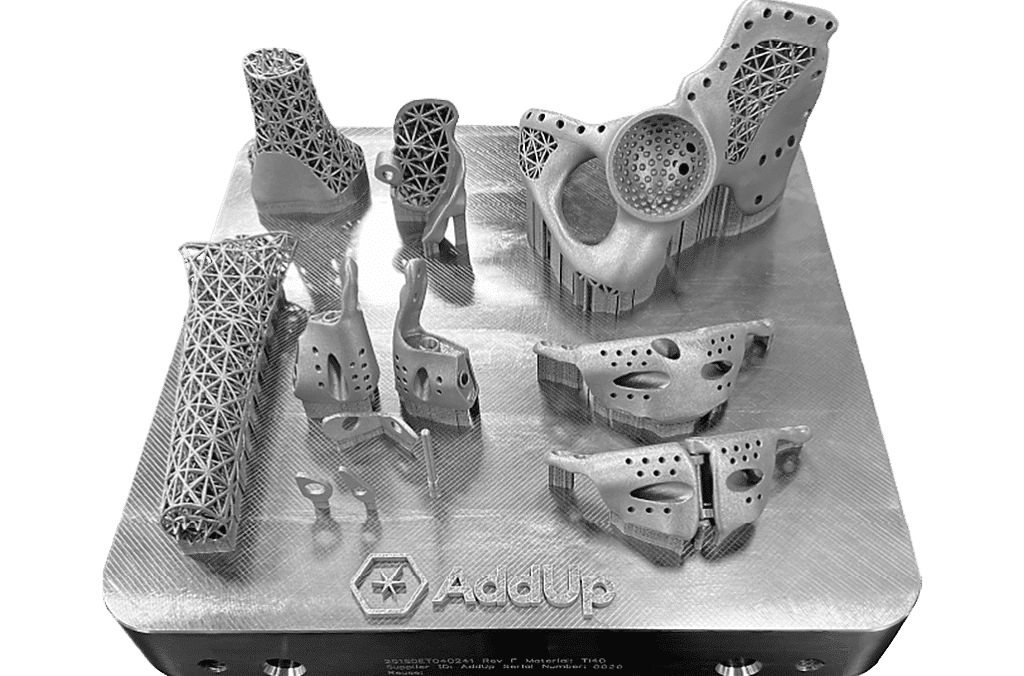
INDUSTRY
Medical
CHALLENGE
As the world around us becomes more personalized, medicine is no different. To keep up, off the shelf solutions will become obsolete and personalized solutions will become the norm. How will the industry handle this customized changed from a manufacturing standpoint?
KEY BENEFITS
- a net savings of US$736 per operation when using additively manufactured PSI (1)
- a decrease in blood loss (of 44.72 mL) when using additively manufactured PSI (2)
- a decrease in hospital stay (0.39-day decrease) (2)
Custom Shape
Performance
Integrated Features
This case study highlights the advantages of using additive manufacturing (AM) for Patient Specific Implants (PSI) in the orthopedic industry. By shifting from traditional manufacturing to AM, orthopedic OEMs can meet the demand for personalized medicine and tailored solutions for patients.
History
Before the invention of industrial 3D Printing, all standard-line and even some Patient Specific Implants (PSI) were traditionally manufactured. Typically, this included manufacturing methods like casting and forging and CNC machining out of bar stock. These implants must be machined from a single piece of material (most likely titanium or stainless steel). This is a particularly expensive and technically sophisticated process. This leads to the PSI being costly and with an increased lead time.
When using AM, there are many ways that these devices can be cleared through the FDA. The easiest and most used is a 510k. This verifies a “build envelope” and ensure the PSI is functionally equivalent or better that the standard line implant. Another option is a Custom Device Exemption. This is an option that limits the manufacturing of a particular device type to 5 units per year(3). Humanitarian Use Devices (HUD) are medical devices intended to benefit patients in the treatment or diagnosis of a disease or condition that affects or is manifested in not more than 8,000 individuals in the United States per year. A Humanitarian Device Exemption (HDE) is a subset of the HUD. This type of PSI is exempt from the effectiveness requirements of Sections 514 and 515 of the FD&C Act and is subject to certain profit and use restrictions(4). These are the plethora of ways that OEMs and Manufacturers can help get the PSI into the hands of the surgeon.
Challenges
Orthopedic OEMs have been manufacturing standard line implants for mostly the same way since the 1970s. A shift to additively manufactured PSIs will change how surgeons treat their patients and will change the industry as we know it.
Conventional manufacturing, whether it be subtractive or casting and forging, is not inherently designed to make customized solutions. Therefore, the largest challenge will be convincing the OEMs and implant manufactures to change their manufacturing processes to match what the market is demanding. The market is demanding personalized medicine, and this will come in the form of PSI in the orthopedic industry.
The patients will need to work with surgeons to ensure that they receive the most tailored solution to their condition. This will also require cooperation from the hospitals and insurance companies to provide support for this industrial change. PSI can be cheaper and more beneficial to the patient, but as the technological shift occurs, PSI will most likely be more expensive. It will be up to the user, patient and surgeon, to vote with their wallet and the equipment they use to enable this technology to flourish.
SOLUTIONS
AddUp is uniquely equipped to help the industry shift from standard line implants to patient specific implants.
The FormUp 350 is built for serial production from the ground up. It can handle varying different complex geometries from fine detailed lattice that promotes osteointegration to a large semipelvis. These types of cases can all be built on a single build allowing for greater efficiency and throughput.
The modular build plate helps the manufacturer to adapt to surgical cases of any size and shape. This allows for greater efficiencies from each build. Efficiency will be key to the shift from standard product line to patient specific implants happening. As the population ages and a larger number of people live longer, there will be more and more surgeries. If medicine continues down the path of personalization, the FormUp 350 will be there to meet the demand of serial production of patient specific implants.
Lot traceability is inherently enhanced, and implants can get on to their next process faster without the need to wait on the remainder of the build. This means that each surgical case can go its own way closer to the beginning of the supply chain. A wider range of surgical implants can be produced on the same build since each implant is not subject to as many of the same processes. These further decreases lead times as PSI are especially sensitive to the amount of time between the CT scan to the surgery. Any amount of time between CT scan to surgery allows the bone’s anatomy to change as the patient continues living their day-to-day life. The less amount of time from scan to surgery, the better possible outcome for the patient; giving surgeon’s confidence that they have the correct tools for the job to best improve the patient’s life.
The Results
Using a Total Knee Arthroplasty (TKA) example, using a PSI manufactured via AM results in a net savings of $736 when compared to traditionally manufactured implants, thanks to a shorter operating time and fewer instrument trays required.(1) Patients and hospitals also reap the benefit of shorter operating room times, reduced by 20.4 min(1) when compared to traditionally manufactured implants.
It’s also been proven that a significant difference in blood loss occurs (decreased by 44.72 mL)(2) Lastly, a decreased hospital stay (0.39 day decrease) provides a significant benefit to both the hospital system and the patient. (2)
Using additively manufactured PSIs such as knee femoral and tibial components or acetabular hip cups, provide improved accuracy of biomechanical implant alignment(1), resulting in improved patient care and better patient outcomes.
References
1. Haglin, J.M., Eltorai, A.E.M., Gil, J.A., Marcaccio, S.E., Botero-Hincapie, J. and Daniels, A.H. (2016), Patient Specific Orthopaedic Implants. Orthop Surg, 8: 417- 424. https://doi.org/10.1111/os.12282
2. Schwarzkopf, Ran, et al. “Surgical and functional outcomes in patients undergoing total knee replacement with patient-specific implants compared with “off-the-shelf” implants.” Orthopaedic journal of sports medicine
3.7 (2015): 2325967115590379
3. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/custom-device-exemption