INDUSTRY
Medical
CHALLENGE
Bringing Laser Powder Bed Fusion (LBPF) to Total Hip Replacements to reduce production costs using a multi laser system and a larger build plate
KEY BENEFITS
- Maximum throughput with 78% OEE
- No supports = reduced post processing = lower part cost
- Reduced lead time
- Fine feature resolution and optimal osseointegration for better patient outcomes
Integrated Features
Reduced Lead Time
No Support
Performance
History
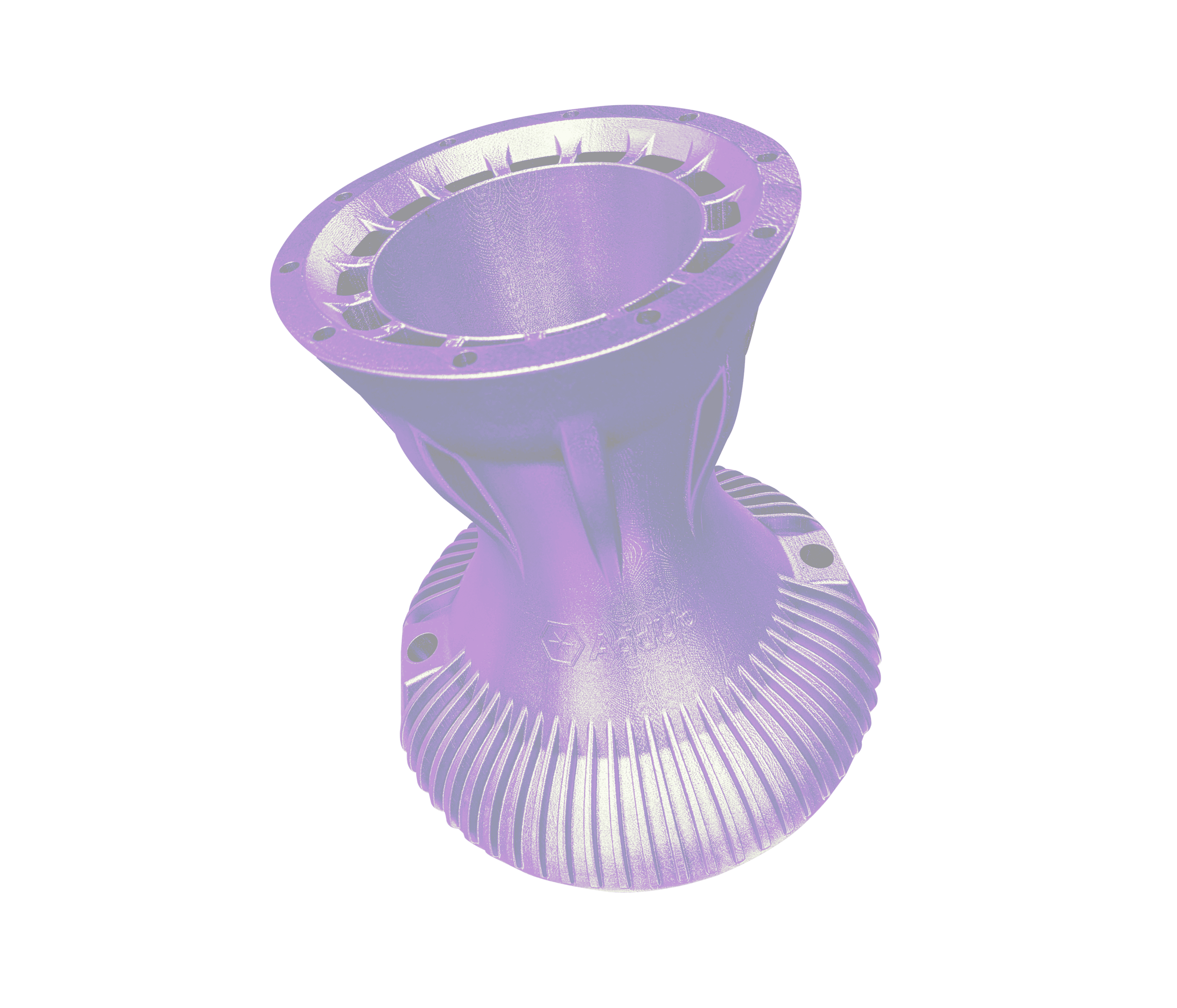
Acetabular cups are used during total hip replacements to sit against the native bone of the illum and articulates with the femur through the hip stem. Inside of the hip cup there sits a liner that connects with the head of the stem for articulation of the hip.
Acetabular hip cups are traditionally manufactured by casting and forging. This method has a long turnaround time from order to final product due to the lost wax method. This method creates a sacrificial wax mold that a shell is formed around. Then the wax is melted out of the shell and the metal of choice is poured into the shell. The shell is then broken to reveal the final part in the metal of choice. Then these acetabular hip cups must have some sort of porous structure applied to them, which is either expensive to manufacture or difficult to validate.
When additively manufactured, the part is typically printed using Electronic Beam Technology (EBM). This manufacturing process uses a stream of electrons guided by a magnetic field to melt layers of powder on top of each other. The EBM technology is subject to unpredictable failures. This is particularly unsatisfactory when multiple hip cups are being stacked onto one another in a single build. This creates a cascading effect where one failed part can result in a large amount of scrap at once. Additionally, it complicates the validation process as each layer must be mechanically validated independently.
Challenges
Casting and forging parts require a large amount of time to manufacture. This method requires foundries that are only justified with large part volumes. The long primary process along with the additional steps creates a bottleneck in the supply chain, leading to increased prices, inventory, and lead times.
Although EBM can be faster than Direct Laser Metal Sintering or Laser Powder Bed Fusion, DMLS/LPBF produces smoother, more accurate parts with no supports. Parts manufactured by EBM technology are less precise and have a higher surface roughness. This results in increased post-processing costs. Rough material on the surface must be traditionally machined away. The medical device industry is specifically sensitive to roughness that can lead to an increase in risk of build failure as the build time is longer. This does not coincide well with a technology that has a lower Overall Equipment Effectiveness (OEE).
STRATEGY
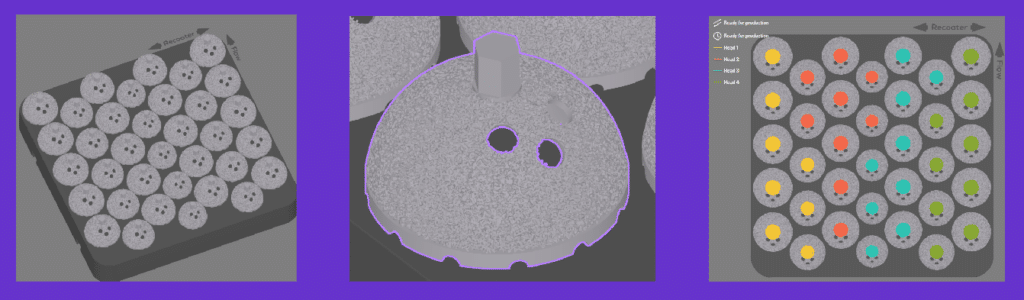
Laser Powder Bed Fusion (LPBF) technology provides a closer net shape part compared to EBM technology. There are also no supports needed in LPBF technology. All of which significantly reduces less post processing, reducing lead times. The FormUp 350 also has a larger build plate with more lasers compared to EBM printers leading to potentially more than double the throughput. AddUp’s FormUp 350 also has a fine feature resolution and a roller recoater which allows for a lattice structure printed with the implant. Lattice structures improve osseointegration which allows for longer lasting implants and better patient outcomes.

RESULTS
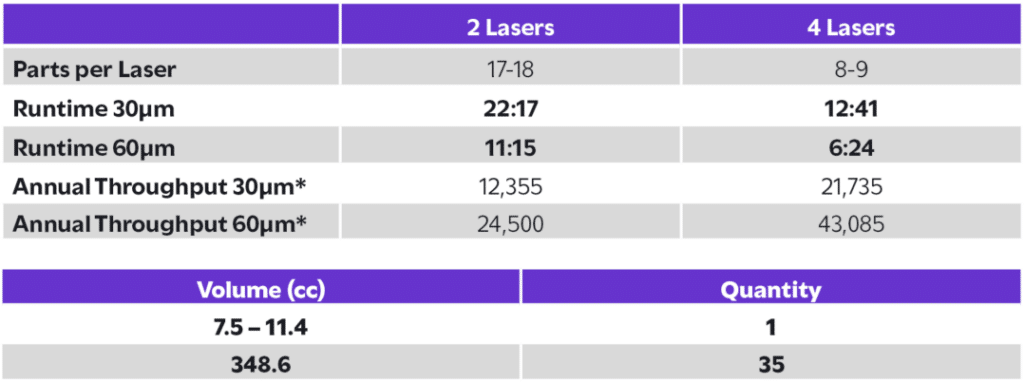
The FormUp 350 produced 35 acetabular cups in under 13 hours using 30 µm Ti6Al4V ELI, achieving a 100% part yield with no failures. Compared to EBM, the FormUp 350 demonstrated an 18% faster cycle time and supported annual throughputs of up to 43,085 cups per machine with a 4-laser, 60 µm configuration, nearly doubling EBM’s capacity. No support structures were required, reducing post-processing labor and cost while maintaining high density and surface quality. This performance translates to shorter lead times, lower part costs, and consistent, repeatable output that meets the demanding standards of medical manufacturing, evidence that serial production of critical implants is not just feasible, but proven.